Estudios clínicos disponibles
COLORECTAL CANCER
BESPOKE study of ctDNA guided therapy in colorectal cancer (CRC) Signatera detects and tracks circulating tumor DNA (ctDNA) using a personalized, tumor-informed assay
STUDY DESCRIPTION
This is a first prospective, multi-center clinical study examining the role of ctDNA in: MRD Assessment | Adjuvant Treatment Guidance | Recurrence Monitoring. BESPOKE CRC will measure changes in treatment decisions and clinical outcomes based on the use of Signatera in patients with stage II and III colorectal cancer. The study will enroll at least 1,000 patients. Natera and its collaborators will collect clinical utility and outcomes data on enrolled patients for two years.
INCLUSION CRITERIA
- Pathologic Stage II and III
- Has residual surgically resected FFPE tissue from adenocarcinoma of the colon or rectum
- ECOG performance status ≤ 2
- Clinically eligible for chemotherapy
- Able to tolerate collection of up to 30 mL of blood via venipuncture
- 18 years or older
- Able to provide written informed consent
COLORECTAL CANCER (Advanced or Metastatic)
A Phase 1b/2, Open-Label Study of Amivantamab Monotherapy and in Addition to Standard-of-Care Chemotherapy in Participants With Advanced or Metastatic Colorectal Cancer
STUDY DRUG
- Amivantamab
- Fluorouracil
- Leucovorin
- Oxaliplatin
- Irinotecan
INCLUSION CRITERIA
- Participant must have been previously diagnosed with histologically or cytologically confirmed unresectable or metastatic adenocarcinoma of the colon or rectum
- For Phase 1 dose confirmation cohorts (Cohorts Ph1b-D and Ph1b-E): Participant must have evaluable disease. For Phase 2 dose expansion cohorts (Cohorts D and E): Participant must have measurable disease according to Response Criteria in Solid Tumors (RECIST) Version 1.1. If only one measurable lesion exists, it may be used for the screening biopsy as long as baseline tumor assessment scans are performed greater than or equal to (>=) 7 days after the biopsy
- Participant must have a tumor lesion amenable for biopsy and agree to mandatory protocol-defined screening biopsy
COLORECTAL CANCER (Advanced and KRAS G12C Mutation)
A Randomized Phase 3 Study of MRTX849 in Combination With Cetuximab Versus Chemotherapy in Patients With Advanced Colorectal Cancer With KRAS G12C Mutation With Disease Progression On or After Standard First-Line Therapy
STUDY DRUG
- MRTX849
- Cetuximab
- MFOLFOX6 Regimen
- FOLFIRI Regimen
INCLUSION CRITERIA
- Histologically confirmed diagnosis of colorectal carcinoma with KRAS G12C mutation in tumor tissue.
- Prior receipt of 1st line treatment in advanced CRC with a fluoropyrimidine-based chemotherapy regimen containing either oxaliplatin or irinotecan, and radiographically documented progression of disease on or after treatment.
COLORECTAL CANCER
A Phase 1b/2, Randomized, Open-Label Study Investigating the Efficacy and Safety of LBL-007 Plus Tislelizumab in Combination With Bevacizumab Plus Capecitabine Versus Bevacizumab Plus Capecitabine as Maintenance Therapy in Patients With Unresectable or Metastatic Microsatellite Stable/Mismatch Repair Proficient Colorectal Cancer
INCLUSION CRITERIA
- Colorectal adenocarcinoma with unresectable or metastatic disease (Stage IV)
- No prior systemic therapy for CRC in the metastatic setting except for the induction treatment of the first-line therapy.
- Completed induction therapy (defined as 8 cycles or 4 months) with FOLFOX/FOLFIRI/FOLFIRINOX + Bevacizumab OR CapeOX + Bevacizumab with overall response of stable disease or better.
COLORECTAL CANCER
A Phase 2, Randomized, Open-Label Study Evaluating the Safety and Efficacy of Magrolimab in Combination With Bevacizumab and FOLFIRI Versus Bevacizumab and FOLFIRI in Previously Treated Advanced Inoperable Metastatic Colorectal Cancer (mCRC)
INCLUSION CRITERIA
- Adenocarcinoma mCRC
- Previously treated patients with inoperable mCRC who have progressed on or after 1 prior systemic therapy and who are ineligible for checkpoint inhibitor therapy.
- Prior therapy must have included chemotherapy based on 5- FU with oxaliplatin and either bevacizumab or cetuximab or panitumumab.
CHOLANGIOCARCINOMA
Phase 1b/2a Study of GNS561 in Combination with Trametinib in Advanced KRAS Mutated Cholangiocarcinoma
STUDY DESCRIPTION
This is an open label, multi-center, Phase 1b/2a study to evaluate the safety, pharmacokinetics (PK), pharmacodynamics (PD) and efficacy of GNS561 given in combination with trametinib, in 21-day cycles in a standard 3 + 3 design, in patients with advanced KRAS mutated CCA after failure of SoC first line therapy.
INCLUSION CRITERIA
- Adult patients with histologically or cytologically confirmed cholangiocarcinoma at the time of diagnosis with documented KRAS mutation.
- Locally advanced unresectable disease not amenable to local treatment with surgery or radiotherapy, OR metastatic disease.
- Patients must have received at least one prior regimen and have evidence of progressive disease following prior regimen.
- Measurable disease on CT or MRI by RECIST; at least 1 lesion not previously treated.
- ECOG performance status 0-1
HEPATOCELLULAR CARCINOMA (Advanced/Metastatic)
A Phase1/2, Safety Confirmation and Double-blind, Placebo-controlled, Randomized Study of Relatimab in Combination with Nivolumab and Bevacizumab in Treatment-naïve Advanced/Metastatic Hepatocellular Carcinoma (RELATIVITY-106)
STUDY DRUG
- Relatlimab
- Nivolumab
- Bevacizumab
INCLUSION CRITERIA
- Histologically confirmed advanced/metastatic hepatocellular carcinoma (HCC)
- Naïve to systemic therapy for advanced/metastatic HCC (prior neo-adjuvant or adjuvant immunotherapy is permitted if recurrence occurs ≥ 6 months after treatment completion and the case is discussed with BMS medical team)
- Child-Pugh score of 5 or 6 (ie, Child-Pugh A)
NON-SMALL CELL LUNG CANCER (NSCLC) AND ADVANCED SOLID TUMORS
Phase 1 / 2 Multicenter Study of the Safety, Pharmacokinetics, and Preliminary Efficacy of APL-101 in Subjects With Non-Small Cell Lung Cancer With c-Met EXON 14 Skip Mutations and c-Met Dysregulation Advanced Solid Tumors.
STUDY DRUG
- APL-101
INCLUSION CRITERIA
- For Phase 1, histologically and / or cytological confirmed unresectable or metastatic solid malignancy, refractory to standard therapies with no more than three prior lines of therapy.
- For Phase 2, five cohorts will be enrolled: Cohort A-1: NSCLC EXON 14 skip mutation (c-Met naïve) for first line treatment, Cohort A-2: NSCLC EXON 14 skip mutation (c-Met naïve) pretreated subjects with no more than 3 lines of prior therapy, Cohort B: NSCLC EXON 14 skip mutation (c-Met experienced; radiographic progression on prior c-Met inhibitor), Cohort C: basket of tumor types with c-Met high level amplification (NSCLC EXON 14 skip mutation excluded), Cohort D: basket of tumor type with c-Met fusions.
- Local/archival result (tissue and/or plasma) of a positive c-Met dysregulation is required (except in Cohort A-1 in the US).
- Measurable disease according to RECIST v1.1. (or relevant criteria per tumor type).
- For all prior anticancer treatment, including radiotherapy, chemotherapy or targeted agents or hormonal therapy, a duration of more than 30 days or 5 half-lives of the agents used, whichever is shorter, must have elapsed, and any encountered toxicity must have resolved to levels meeting all the other eligibility criteria prior to the first dose of study treatment.
- No planned major surgery within 4 weeks of first dose of APL-101.
SOLID TUMORS (ADVANCED AND UNRESECTABLE OR METASTATIC)
Tumor-Agnostic Precision Immunooncology and Somatic Targeting Rational for You (TAPISTRY) Phase II Platform Trial
STUDY DRUG
- Entrectinib
- Alectinib
- Atezolizumab
- Ipatasertib
- Trastuzumab emtansine
- Idasanutlin
- Inavolisib
- Belvarafenib
- Pralsetinib
INCLUSION CRITERIA
- Histologically or cytologically confirmed diagnosis of advanced and unresectable or metastatic solid malignancy.
- Measurable disease as defined by Response Evaluation Criteria in Solid Tumors, Version 1.1 (RECIST v1.1), Response Assessment in Neuro-Oncology (RANO) criteria, or International Neuroblastoma Response Criteria (INRC).
- Disease progression on prior treatment, or previously untreated disease with no available acceptable treatment.
- Adequate recovery from most recent systemic or local treatment for cancer.
- Life expectancy >= 8 weeks.
SOLID TUMORS IN BREAST, TRIPLE NEGATIVE BREAST, ENDOMETRIAL AND OVARIAN CANCER (ADVANCED OR METASTATIC)
A Phase 1 Dose Escalation and Expansion study to Evaluate the Safety, Tolerability, Pharmacokinetic, Pharmacodynamic, and Anti-Tumor activity of PF-07260437 in Advanced or Metastatic Solid Tumors
STUDY DRUG
- PF-07260437
INCLUSION CRITERIA
- Part 1: Histological/cytological diagnosis of selected locally advanced or metastatic breast cancer, endometrial cancer and ovarian cancer
- Part 2A:In second line or more, participants with histological/cytological diagnosis of locally advanced or metastatic HR+ HER2- breast cancer showing high B7-H4 expression
- Part 2B: In second line or more participants with histological or cytological diagnosis of locally advance or metastatic HR+ Her2- breast cancer or triple negative breast cancer (TNBC) with no biomarker pre-selection
- Part 2C: In second line or more participants with histological diagnosis of locally advance or metastatic triple negative breast cancer with high B7-H4 expression
SOLID TUMORS (ADVANCED MALIGNANT AND HARBORING KRS OR EGFR MUTATIONS)
A Phase 1, Open-Label, Dose Escalation of HBI-2376 in Patients With Advanced Malignant Solid Tumors Harboring KRAS or EGFR Mutations
STUDY DRUG
- HBI-2376
INCLUSION CRITERIA
- Male or female at least 18 years of age at the time of signing the ICF prior to initiation of any study specific activities/procedures
- Advanced malignant solid tumors with KRAS or EGFR mutations diagnosed by histology or cytology
- Relapsed or refractory to, or intolerant of, or refuse approved or standard of care established therapy known to provide clinical benefit for disease
- At least 1 measurable target lesion that meets the definition of RECIST v1.1
- Demonstrate adequate organ function
- Must be able to swallow oral medications and must not have gastrointestinal abnormalities that significantly affect drug absorption
SOLID TUMORS (ADVANCED MALIGNANT AND HARBORING KRAS G12C MUTATION)
A Phase 1, Open Label, Dose Escalation of HBI-2438 in Patients With Advanced Malignant Solid Tumors Harboring KRAS G12C Mutation
STUDY DRUG
- HBI-2438
INCLUSION CRITERIA
- Male or female at least 18 years of age at the time of signing the ICF prior to initiation of any study specific activities/procedures
- Advanced malignant solid tumors with KRAS G12C mutation- as determined by genetic testing
- Must have failed or refused standard of care therapy, are not eligible for standard of care therapy, or cannot benefit from standard of care therapy, in the opinion of the Investigator
- At least 1 measurable target lesion that meets the definition of RECIST v1.1
- Demonstrate adequate organ function
- Expected survival time > 3 months in the opinion of the investigator
- Must be able to swallow oral medications and must not have gastrointestinal abnormalities that significantly affect drug absorption
SOLID TUMORS IN LUNG OR COLORECTAL (LOCALLY ADVANCED OR METASTATIC; LUNG OR CRC COHORTS) *
A Phase 1 First-in Human, Multi-Center, Open Label Dose-Escalation Study to Determine the Safety, Tolerability, Pharmacokinetics and RP2D of ABBV-151 as a Single Agent and in Combination with ABBV-181 in Subjects with Locally Advanced or Metastatic Solid Tumors
STUDY DESCRIPTION
The study will determine the recommended Phase 2 dose (RP2D) of ABBV-151 administered as monotherapy and in combination with ABBV-181 as well as to assess the safety, pharmacokinetics (PK), and preliminary efficacy of ABBV-151 alone and in combination with ABBV-181.
STUDY DRUG
- ABBV-155
- Budigalimab
INCLUSION CRITERIA
- For Dose Escalation only: Participants with an advanced solid tumor who are considered refractory to or intolerant of all existing therapy(ies) known to provide a clinical benefit for their condition. Additionally, participants who have been offered standard therapies and refused, or who are considered ineligible for standard therapies, may be eligible for this study on a case-by-case basis, after discussion with and agreement from the sponsor.
- Subjects with histologically or cytologically confirmed advanced or metastatic NSCLC expression PD-L1 [Tumor Proportion Score (TPS) >/= 50% as determined by an FDA-approved test]
- Have received no prior therapy for advanced disease, with no EGFR or ALK genomic tumor aberration.
- Subjects must consent to provide tumor biopsies collected within 6 months prior to Cycle 1 Day 1 (C1D1) with no intervening therapies. A fresh biopsy must be submitted if archival tissue is unavailable per these specifications.
- CRC participants with microsatellite stable or mismatch repair proficient colorectal adenocarcinoma (as determined by PCR/NGS or IHC, respectively) who have received 1-2 prior chemotherapy regimens and who have refused or are ineligible for other approved therapies.
SOLID TUMORS (Advanced)
A Phase 1 First in Human Study Evaluating Safety, Pharmacokinetics and Efficacy of ABBV-400 in Adult Subjects With Advanced Solid Tumors
STUDY DRUG
- ABBV-400
INCLUSION CRITERIA
- For Part 1 only – history of advanced solid tumor that has progressed on all standard of care therapy and are not amenable to surgical resection or other approved therapeutic options that have demonstrated clinical benefit.
- For Part 2 only – history of advanced non-squamous wtEGFR or mutEGFR Non-Small Cell Lung Cancer (NSCLC) and are c-Met positive that has progressed after treatment per the protocol.
- Should have no more than 2 lines of prior cytotoxic chemotherapy excluding adjuvant therapy and must have advanced NSCLC that is not amenable to surgical resection or other approved therapeutic options that have demonstrated clinical benefit.
SOLID TUMORS (Advanced and KRAS G12C Mutation)
A Phase 1/2 Multiple Expansion Cohort Trial of MRTX849 in Patients With Advanced Solid Tumors With KRAS G12C Mutation KRYSTAL-1
STUDY DRUG
- MRTX849
- Pembrolizumab
- Cetuximab
- Afatinib
INCLUSION CRITERIA
- Histologically confirmed diagnosis of a solid tumor malignancy with KRAS G12C mutation.
- Unresectable or metastatic disease.
- Standard treatment is not available or patient declines; first-line treatment for NSCLC for certain cohorts.
- Adequate organ function.
MUSCLE INVASIVE BLADDER CANCER CHEMORADIOTHERAPY +/- PEMBROLIZUMAB
A Phase 3, Randomized, Double-blind, Placebo-controlled Clinical Trial to Study the Efficacy and Safety of Pembrolizumab (MK-3475) in Combination With Chemoradiotherapy (CRT) versus CRT Alone in Participants with Muscle-invasive Bladder Cancer (MIBC) (KEYNOTE-992)
STUDY DESCRIPTION
This is a Phase 3, randomized, Double-blind, Placebo-controlled Clinical Trial to Study the Efficacy and Safety of Pembrolizumab (MK-3475) in Combination With chemoradiotherapy (CRT) versus CRT Alone in Participants with Muscle-invasive bladder Cancer (MIBC) (KEYNOTE-992).
STUDY DRUG
- Pembrolizumab
- Conventional Radiotherapy
- Cisplatin
- Fluororacil (5-FU)
- Mitomycin (MCC)
- Gemcitabine
- Pembrolizumab
INCLUSION CRITERIA
- Male and female participants at least 18 years of age with MIBC clinical stage T2-T4aN0M0 who elect to receive CRT
- Has a histologically confirmed diagnosis of muscle-invasive bladder cancer (MIBC) with predominant urothelial histology.
- Has clinically non-metastatic bladder cancer (N0M0).
- Has planned and is eligible to receive chemoradiotherapy (CRT) and one of the protocol-specified radiosensitizing chemotherapy regimens.
NON-MUSCLE INVASIVE BLADDER CANCER
A Phase II Clinical Trial to Study the Efficacy and Safety of Pembrolizumab (MK-3475) and Pembrolizumab in Combination with Other Investigational Agents in Subjects with High-risk Non-muscle-Invasive Bladder Cancer (NMIBC) Unresponsive to Bacillus Calmette-Guerin (BCG) Therapy
STUDY DESCRIPTION
Phase II Clinical Trial to study the efficacy and safety of MK7684A or MK4280A in participants with High-risk Non-muscle Invasive Bladder Cancer (NMIBC) Unresponsive to Bacillus Calmette-Guerin (BCG) therapy and non-eligible or have elected not to undergo a radical cystectomy.) (KEYNOTE-057).
STUDY DRUG
- Pembrolizumab
- MK-7684
- MK-4280
INCLUSION CRITERIA
- Have a histologically-confirmed diagnosis of high risk non-muscle-invasive (T1, High Grade Ta and/or CIS) transitional cell carcinoma of the bladder.
- Participants with tumors of mixed transitional/non-transitional cell histology are allowed, but transitional cell carcinoma must be the predominant histology.
- Participants with T1 must have undergone complete TURBT
- Have been treated with adequate BCG therapy and have developed high-risk NMIBC that is unresponsive to BCG therapy.
- ECOG performance status 0, 1, 2
METASTATIC CR PROSTATE CANCER
A Study of Nivolumab or Placebo in Combination With Docetaxel in Men With Advanced Castration-resistant Prostate Cancer Urothelial NCT04100018
STUDY DESCRIPTION
The purpose of this study is to test the safety and effectiveness of nivolumab with docetaxel in men with advanced castration resistant prostate cancer who have progressed after second generation hormonal manipulation.
INCLUSION CRITERIA:
- Histologic confirmation of adenocarcinoma of the prostate without small cell features
- Current evidence of metastatic disease
- Ongoing androgen deprivation therapy (ADT) with a gonadotropin-releasing hormone (GnRH) analogue or bilateral orchiectomy
- Documented prostate cancer progression criteria within 6 months prior to screening
- Chemotherapy-naïve for metastatic castration- resistant prostate cancer (mCRPC),
- Sufficient tumor samples from either a fresh biopsy (obtained during screening) or archival tumor tissue in the form of formalin-fixed paraffin-embedded (FFPE) block or unstained tumor tissue slides
UROTHELIAL CARCINOMA
Study of Oral Infigratinib for the Adjuvant Treatment of Subjects With Invasive Urothelial Carcinoma With Susceptible FGFR3 Genetic Alterations NCT04197986
STUDY DESCRIPTION
This is a Phase 3 multicenter, double-blind, randomized, placebo-controlled study to evaluate the efficacy of giving an oral targeted FGFR1-3 inhibitor, infigratinib, as adjuvant treatment following surgery in adult subjects with invasive urothelial carcinoma and susceptible FGFR3 genetic alterations (mutations, and gene fusions or translocations [ie, rearrangements) who have disease that is considered at high risk for recurrence with surgery alone.
INCLUSION CRITERIA:
- Have histologically or cytologically confirmed, invasive urothelial carcinoma with susceptible FGFR3 alterations
- If the patient received neoadjuvant chemotherapy, pathologic stage at surgical resection must be AJCC Stage ≥ ypT2 and/or yN+
- If the patient did not receive neoadjuvant chemotherapy:- Must be ineligible to receive cisplatin-based adjuvant chemotherapy per the Galsky criteria- Pathologic stage must be AJCC Stage ≥pT2 pN0-2 M0 (post-lymphadenectomy or no lymphadenectomy [pNx]) for upper tract disease- Pathologic stage should be AJCC Stage ≥pT3 or pN+ (bladder cancer)
- Have Eastern Cooperative Oncology Group (ECOG) performance status of ≤2
- Patients must have no evidence of metastatic disease
WALDENSTRÖM MACROGLOBULINEMIA
A Phase 4, Observational Study Evaluating the Efficacy and Safety of the Bruton Tyrosine Kinase (BTK) Inhibitor Zanubrutinib in Patients With Waldenström Macroglobulinemia
OBSERVATIONAL STUDY – STUDY DRUG
- Zanubrutinib
INCLUSION CRITERIA
- Clinical and definitive histologic diagnosis of WM
- Measurable disease, as defined by a serum immunoglobulin M (IgM) level > 0.5 g/dL at the time of zanubrutinib initiation
- Started treatment with zanubrutinib, has been treated with zanubrutinib, or is planned to be prescribed zanubrutinib for the treatment of WM
- Bone marrow specimens with central MYD88 test results of:
- Cohort 1: MYD88 L265P mutation; enrollment of TN participants will be stopped in each racial and ethnic participant group when the required numbers of participants in the group are met
- Cohort 2: non-L265P MYD88 mutation(s) and MYD88WT
MULTIPLE MYELOMA
A Post-authorization Safety Study to Evaluate the Incidence of and Risk Factors for Severe and Fatal Infusion-related Reactions in Participants Treated with Daratumumab (Intravenous or Subcutaneous)
DESCRIPTION
This single-arm, prospective observational study aims to assess the risk of severe (Grades 3 to 4) and fatal (Grade 5) infusion-related reactions (IRRs) in participants treated with intravenous (IV) or subcutaneous (SC) daratumumab for the treatment of multiple myeloma (MM) in the clinical practice setting and characterize potential risk factors.
OBSERVATIONAL STUDY – STUDY DRUG
- Daratumumab
INCLUSION CRITERIA
- Participant must be aged at least 18 years or legal age of consent in the jurisdiction that the study is conducted.
- Participants must be starting daratumumab treatment for the first time in accordance with the approved label.
CLASSICAL HODGKIN LYMPHOMA
A Study of BGB-A317 as Monotherapy in Relapsed or Refractory Classical Hodgkin Lymphoma NCT04318080
STUDY DESCRIPTION
The study is to evaluate the efficacy of BGB-A317 assessed by Independent Review Committee (IRC) in participants with relapsed or refractory classical Hodgkin lymphoma (cHL), as measured by Overall Response Rate (ORR) per the Lugano Classification.
INCLUSION CRITERIA:
- ≥ 18 years of age at time of informed consent
- Histologically confirmed relapsed or refractory cHL (biopsy from diagnosis or at any relapse is acceptable)
- Participants must have measurable disease defined as ≥ 1 nodal lesion that is > 1.5 cm in the longest diameter, or ≥ 1 extra-nodal lesion (e.g. hepatic nodules) that is > 1 cm in the longest diameter
- Life expectancy ≥ 12 weeks
LOCALLY ADVANCED OR METASTATIC SOLID TUMORS
A Study to Determine the Safety, Tolerability, Pharmacokinetics, and Preliminary Efficacy of ABBV-927 With ABBV-368, Budigalimab (ABBV-181) and/or Chemotherapy in Participants With Locally Advanced or Metastatic Solid Tumors NCT03821935
STUDY DESCRIPTION
A study evaluating the safety, pharmacokinetics (PK), pharmacodynamics, and preliminary efficacy of ABBV-927 with ABBV-368, Budigalimab (ABBV-181) and/or chemotherapy in participants with selected solid tumors. This study consists of 2 main parts, a dose- escalation phase and a dose-expansion phase.
INCLUSION CRITERIA:
- Adequate liver, kidney and hematology function as demonstrated by laboratory values detailed in the study protocol
- An Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1
ADVANCED NON-SMALL CELL LUNG CANCER
A Study of Lazertinib as Monotherapy or in Combination With JNJ-61186372 in Participants With Advanced Non-small Cell Lung Cancer NCT03649971
STUDY DESCRIPTION
The purpose of this study is to confirm the tolerability of recommended Phase 2 dose (RP2D) of lazertinib (Phase 1), to determine the tolerability and identify the recommended Phase 2 combination dose of lazertinib when combined with JNJ-61186372 (Phase 1b), to characterize the safety and tolerability of lazertinib and JNJ 61186372 combinations at the RP2CD in participants with advanced NSCLC with documented EGFR mutation (Phase 1b expansion cohorts) and to estimate the antitumor activity of lazertinib and JNJ 61186372 combinations at the RP2CD in participants with advanced NSCLC with documented EGFR mutation (Phase 1b expansion cohorts).
INCLUSION CRITERIA:
- Histologically or cytologically confirmed non-small cell lung cancer (NSCLC) with previously epidermal growth factor receptor (EGFR) mutation (identified locally in a Clinical Laboratory Improvement Amendments [CLIA]-certified laboratory [or equivalent]) that is metastatic or unresectable, and have progressed after standard of care front-line therapy, and exhausted available options with targeted therapy.
- Evaluable disease
- Eastern Cooperative Oncology Group (ECOG) performance status grade of 0 or 1
- Participants must meet the study protocol defined laboratory criteria without having a history of red blood cell transfusion, platelet transfusion, or granulocyte-colony stimulating factor support within 7 days prior to the date of the test
- A woman of childbearing potential: Must have a negative serum beta human chorionic gonadotropin at screening; Must agree not to breast-feed during the study and for 6 months after the last dose of study intervention. Must agree not to donate eggs (ova, oocytes) for the purposes of assisted reproduction during the study and for 6 months after receiving the last dose of study intervention
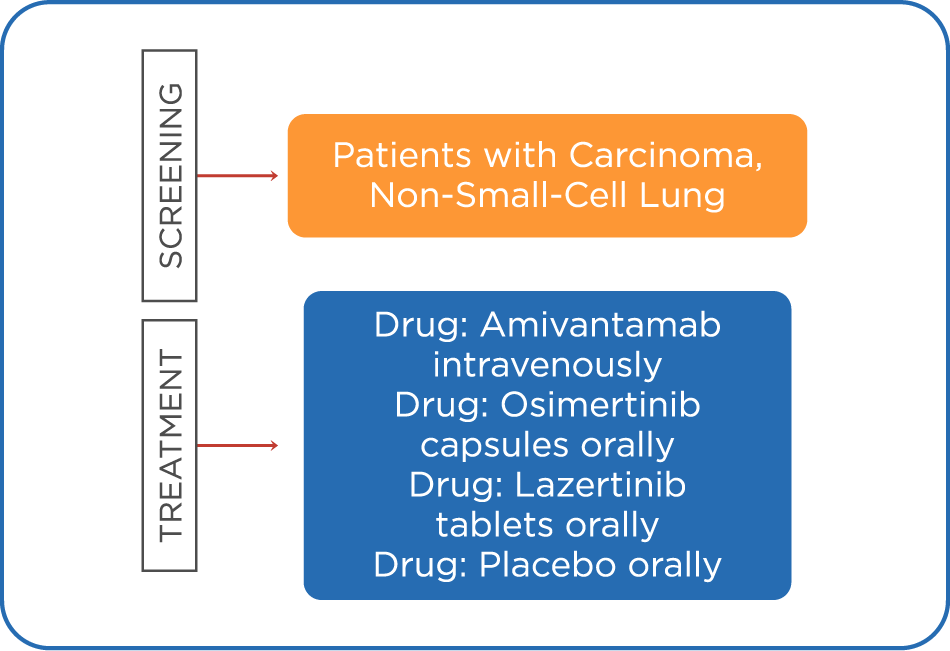
LOCALLY ADVANCED OR METASTATIC NON-SMALL CELL LUNG CANCER
A Study of Amivantamab and Lazertinib Combination Therapy Versus Osimertinib in Locally Advanced or Metastatic Non-Small Cell Lung Cancer (MARIPOSA) NCT04487080
STUDY DESCRIPTION
The purpose of this study is to assess the efficacy of the amivantamab and lazertinib combination, compared with osimertinib, in participants with epidermal growth factor receptor (EGFR) mutation (Exon 19 deletions [Exon 19del] or Exon 21 L858R substitution) positive, locally advanced or metastatic non-small cell lung cancer (NSCLC).
INCLUSION CRITERIA:
- Participant must have histologically or cytologically confirmed, locally advanced or metastatic non-small cell lung cancer (NSCLC) not amenable to curative therapy
- Participant must have a tumor that was previously determined to have exon 19 deletions (Exon 19del) or Exon 21 L858R substitution
- Unstained tumor tissue (in a quantity sufficient to allow for central analysis of epidermal growth factor receptor (EGFR) mutation status, see Laboratory Manual) and blood (for circulating tumor deoxyribonucleic acid [ctDNA], digital droplet polymerase chain reaction [ddPCR], and pharmacogenomic analysis), both collected at or after the diagnosis of locally advanced or metastatic NSCLC, must be provided
- Any toxicities from prior anticancer therapy must have resolved to common terminology criteria for adverse events (CTCAE) Grade 1 or baseline level
- Participant must have at least 1 measurable lesion, according to response evaluation criteria in solid tumors (RECIST) v1.1 that has not been previously irradiated. Measurable lesions should not have been biopsied during screening, but if only 1 non-irradiated measurable lesion exists, it may undergo a diagnostic biopsy and be acceptable as a target lesion, provided the baseline tumor assessment scans are performed at least 14 days after the biopsy
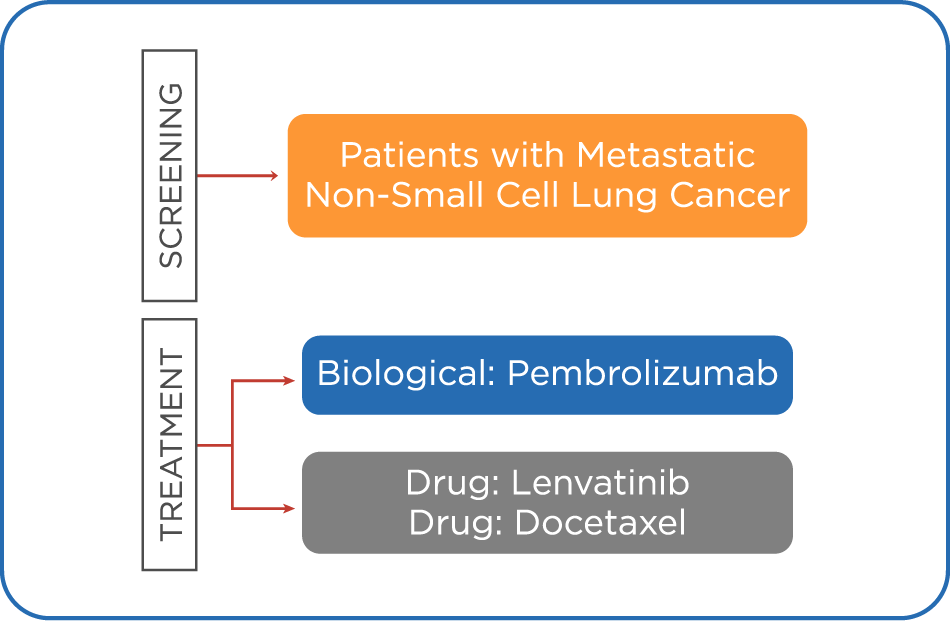
METASTATIC NON-SMALL CELL LUNG CANCER (NSCLC)
Efficacy and Safety of Pembrolizumab (MK-3475) With Lenvatinib (E7080/MK-7902) vs. Docetaxel in Participants With Metastatic Non-Small Cell Lung Cancer (NSCLC) and Progressive Disease (PD) After Platinum Doublet Chemotherapy and Immunotherapy (MK-7902-008/E7080-G000-316/LEAP-008) NCT03906071
STUDY DESCRIPTION
This study will evaluate the efficacy and safety of pembrolizumab (MK-3475) with lenvatinib (E7080/MK-7902) vs. docetaxel in participants with metastatic non-small cell lung cancer (NSCLC) and progressive disease (PD) after platinum doublet chemotherapy and treatment with one prior anti-PD-1/PD-L1 monoclonal antibody (mAb). The primary hypotheses of this study are that pembrolizumab + lenvatinib (compared with docetaxel) prolongs: 1) overall survival (OS); and progression-free survival (PFS) per Response Evaluation Criteria in Solid Tumors Version 1.1 (RECIST 1.1) based on blinded independent central review (BICR).
INCLUSION CRITERIA:
- Has a histologically or cytologically confirmed diagnosis of metastatic squamous or nonsquamous NSCLC (Stage IV: M1a, M1b, M1c)
- Has PD on treatment with one prior anti-PD-1/PD-L1 monoclonal antibody (mAb)
- Has PD during/after platinum doublet chemotherapy for metastatic disease
- Has confirmation that EGFR-, ALK-, or ROS1-directed therapy is not indicated as primary therapy
- Has submitted pre-study imaging that confirmed evidence of PD following initiation of an anti-PD-1/PD-L1 inhibitor
- Has at least 1 measurable lesion by computerized tomography (CT) or magnetic resonance imaging (MRI)
- Has provided tumor tissue for PD-L1 biomarker analysis from an archival sample (defined as: from initial diagnosis of NSCLC and prior to receiving immunotherapy [antiPD-1/PDL1], from the primary lesion or a metastatic lesion)
- Has provided prior to allocation tissue from a newly obtained formalin-fixed sample from a new biopsy (defined as: after completion of immunotherapy [anti-PD-1/PD-L1] and before receiving a randomization number), of a tumor lesion not previously irradiated
CROHN’S DISEASE
A Study of Mirikizumab (LY3074828) in Participants With Crohn’s Disease (VIVID-1) NCT03926130
STUDY DESCRIPTION
This is a Phase 3, Multicenter, Randomized, Double-Blind, Placebo- and Active- Controlled, Treat-Through Study to Evaluate the Efficacy and Safety of Mirikizumab in Patients With Moderately to Severely Active Crohn’s Disease.
INCLUSION CRITERIA:
- Diagnosis of CD or fistulizing CD for ≥ 3 months confirmed by clinical, endoscopic and histological criteria
- Participants with a family history of CRC, personal history of increased CRC risk, age >50 years, or other known risk factor
- Demonstrated intolerance, loss of response or inadequate response to conventional or to biologic therapy for CD
CUTANEOUS SQUAMOUS CELL CARCINOMA (CSCC)
Cemiplimab Survivorship Epidemiology (CASE) NCT03836105
STUDY DESCRIPTION
This is a Cemiplimab Survivorship Epidemiology (CASE) Study.
INCLUSION CRITERIA:
- Patients receiving treatment with cemiplimab for CSCC, or initiating treatment with cemiplimab for CSCC
MELANOMA
A Multicenter, Randomized, Double-Blind Phase 3 Study of HBI-8000 Combined With Nivolumab Versus Placebo With Nivolumab in Patients With Unresectable or Metastatic Melanoma Not Previously Treated With PD-1 or PD-L1 Inhibitors
STUDY DRUG
- HBI
- Nivolumab
INCLUSION CRITERIA
- Histopathologically confirmed diagnosis of non-uveal, Stage III (unresectable), or Stage IV (metastatic) melanoma according to AJCC staging system (8th edition).
- Known BRAF V600 mutation status or consent to BRAF V600 mutation testing before randomization.
- Tumor tissue available for PD-L1 testing at central lab. PD-L1 expression level is required for randomization. In order to be randomized, a patient must be classified as PD-L1 positive or PD-L1 negative according to the following criteria:
- PD-L1 positive (≥ 1% tumor cell membrane staining in a minimum of a hundred evaluable tumor cells) vs
- PD-L1 negative (< 1% tumor cell membrane staining in a minimum of a hundred evaluable tumor cells).
- Note: If an insufficient amount of tumor tissue from an unresectable or metastatic site is available prior to the start of the Screening Phase, patients must consent to allow the acquisition of additional tumor tissue for assessment of the biomarker.
- Have not received anti-PD-1, anti-PD-L1 or other systemic therapy for unresectable or metastatic melanoma, except for the following, provided that the patient has recovered from all treatment-related toxicities:
- BRAF mutation targeting therapy > 4 weeks before administration of Study Treatment.
- Adjuvant or neoadjuvant therapy with PD-1 or PD-L1 inhibitors or anti-CTLA-4) is allowed if disease progression/or recurrence occurred at least 6 months after the last dose and no clinically significant immune related toxicities leading to treatment discontinuation were observed
- Adjuvant interferon therapy must have been completed > 6 weeks before administration of Study Treatment
- Any prior radiotherapy or minor surgery must be completed at least 2 weeks and 1 week respectively before Day 1 dosing and recovered from all treatment related toxicities
BASAL CELL CARCINOMA
Cemiplimab Survivorship Epidemiology (CASE) Study
OBSERVATIONAL STUDY
- Cemiplimab
INCLUSION CRITERIA
- Eligible for treatment with and prescribed cemiplimab for advanced Basal Cell Carcinoma (BCC).
NONALCOHOLIC STEATOHEPATITIS
A Phase 2b Randomized, Double-Blind, Placebo-Controlled, Multicenter Study to Evaluate the Efficacy and Safety of Efinopegdutide (MK-6024) in Adults With Precirrhotic Nonalcoholic Steatohepatitis
STUDY DESCRIPTION
The purpose of this study is to learn how well efinopegdutide works compared to placebo in people who have non-alcoholic steatohepatitis (NASH). Researchers will also learn about the safety and benefit of efinopegdutide and how well people tolerate the medicine. The main goal of the study is to compare how many people taking efinopegdutide or placebo stop showing evidence of NASH without liver scarring getting worse.
STUDY DRUG
- Efinopegdutide
- Semaglutide
INCLUSION CRITERIA
- Histological confirmation of NASH, defined as NAFLD Activity Score (NAS) ≥4 with a score ≥1 point in each component (steatosis, ballooning, and lobular inflammation) AND NASH clinical research network (CRN) fibrosis score of Stage 2 or 3
- No history of Type 2 diabetes mellitus (T2DM) with an A1C ≤9% OR a history of T2DM that is controlled by diet or stable doses of antihyperglycemic agents (AHAs)
SQUAMOUS CELL ANAL CARCINOMA
A Phase 3 Global, Multicenter, Double-Blind Randomized Study of Carboplatin-Paclitaxel With INCMGA00012 or Placebo in Participants With Inoperable Locally Recurrent or Metastatic Squamous Cell Carcinoma of the Anal Canal Not Previously Treated With Systemic Chemotherapy (POD1UM-303/InterAACT 2)
STUDY DRUG
- Carboplatin
- Paclitaxel
- Retifanlimab
INCLUSION CRITERIA
- Able to comprehend and willing to sign a written ICF for the study.
- Histologically or cytologically verified, inoperable locally recurrent or metastatic SCAC.
- No prior systemic therapy other than the following:
- Chemotherapy administered concomitantly with radiotherapy as a radiosensitizing agent is permitted.
- Prior neoadjuvant or adjuvant therapy if completed ≥ 6 months before study entry.
- Able and willing to provide adequate tissue sample and whole blood sample with central testing result prior to randomization. Biopsy for archival samples should have occurred within 6 months prior to randomization.
- If HIV-positive, then must be stable as defined by: a. CD4+ count ≥ 300/μL, b. Undetectable viral load per standard of care assay, c. Receiving antiretroviral therapy (ART/HAART) for at least 4 weeks prior to study enrollment, and have not experienced any HIV-related opportunistic infection for at least 4 weeks prior to study enrollment.